
The development of energy storage solutions, especially for batteries, has been one of the most active industrial fields for many years. The stakes are getting higher to this day with the exponential growth of electric mobility, connected objects and means of communication.
The current context of energy storage
There is one basic principle when talking about electrochemical battery: transforming electrical energy into chemical energy during charging and conversely to restore electrical energy during discharge. This tenet has been used in lead batteries for 150 years. Designed more than 30 years ago, Lithium-ion batteries are as for today the most widespread solution in various sectors. Its very high efficiency (3 times more power than a lead battery of the same weight) and the large number of cycles make it the most efficient solution. Nevertheless, there are many issues to improve existing products: storage capacity, manufacturing cost, safety, ecological cost and of course life expectancy. The work detailed in this application note was focused on these last two points.
Introduction to lithium-ion batteries
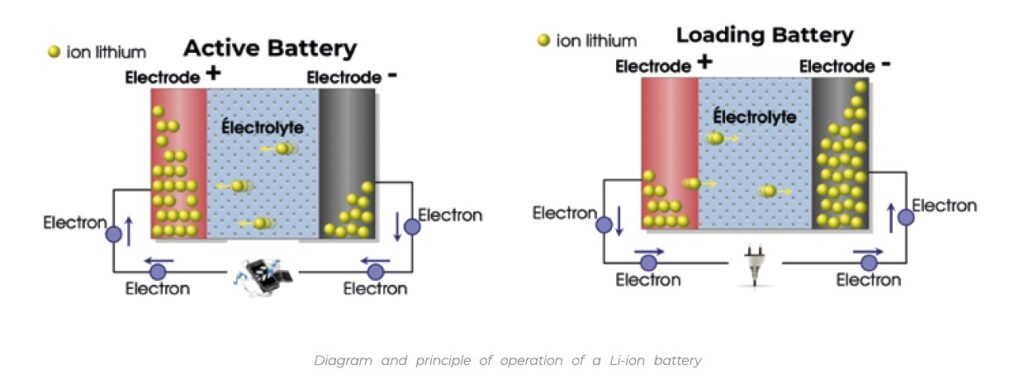
Li-ion batteries are made up of a multitude of cells, each one being able of generating a few volts. Every cell is formed of two electrodes that will exchange ions, lithium in Li-ion batteries. These ions come mainly from the electrolyte salts present in the cell. The Li+ ions migrate from the anode to the cathode during the discharge according to a reversible phenomenon that allows the battery to be recharged as shown in the diagrams below.
The electrodes of Li-ion batteries consist of a porous network of active particles, a conductive additive and a binder. The 3-dimensional distribution of these 3 components plays a crucial role in the battery’s charge capacity.
The analysis on a very small scale of the chemical components of the electrodes is then an essential key to improve the performance of the batteries.
Conventional means of materials analysis often require the destruction of the sample and do not allow the evolution of a battery to be monitored over the charge and discharge cycles. In addition, these techniques provide two-dimensional information only, which is unsuitable for the analysis of a three-dimensional porous network.
X-Ray micro-tomography provides a technical solution to the challenge of understanding the mechanisms of electrode degradation over cycles:
- The acquisition method allows to set up “in situ”
measurements to follow the phenomenon over time by subjecting
the cell to charge/discharge cycles;
- The result is a volume of data that allows the three-dimensional analysis of the electrode on a sub-micron scale.
Learn More?
Please click on ‘Request Application Note’ and we will send you the full application note ‘Energy Storage – a global challenge’.
